Today I intend to do a post explaining the different quantum numbers in simple terms. There is a good chance that in future posts will have a need to refer to quantum numbers – I don’t have something in mind but they are important. My aim is to over time make the site self-sufficient, so when we reference something like a quantum number there is somewhere else the readers can be directed which will give an explanation for those in need.
Principle Quantum Number
The principle quantum number is as it sounds – the important one which if you have taken any basic Chemistry qualification you will know about. The principal quantum number, denoted n, is essentially the average distance that an electron will find itself from the nucleus. It is often referred to as the number of electron shells or orbitals – this isn’t wrong, since when an atom is in its ground state (none of the electrons are excited) the highest principal quantum number will be the number of orbitals that atom has although be warned “orbitals” is misleading. The further from the nucleus of the atom you go, the higher the energy level of the shell. Much like objects will naturally flow to the point of lowest gravitational potential energy electrons will naturally move to the orbital of the lowest energy – as such it should be no surprise that the ground state of the a hydrogen atom, which has one electron has a single electron in the first orbital.

The final question that often arises is why don’t all electrons sit in orbital 1, if this is what electrons naturally want to do? Much the same reason you might leave a crowded underground train and wait for the next one. There are more scientific reasons that this – but consider the fact that as you retreat from the nucleus the size of the orbital increases. Electrons have the same electric charge (along with a host of other clashing qualities), which much like me and other humans, means they refuse to be within a certain proximity. It is not so much that I can’t be that close to other humans, it is more that beyond a certain closeness other arrangements look more attractive. It’s the same deal here – the electron may yearn for the lowest energy arrangement possible, but if it’s overcrowded then the next best will suffice.
Diagrams in any scientific or mathematical discipline are essential – they allow the human mind to model a situation and to rationalise it into something we can understand. However it is important that once we are finished with a diagram or a model, we do a reality check to ensure we haven’t in some way lost sight of the physical situation. The above picture of the atom is what we would consider a Niels Bohr model – very useful for understanding what we have just discussed, but there are two important and fundamental differences with reality – firstly the nucleus in that image is massive. The nucleus is dense and tightly packed – indeed if we were to increase the size of a hydrogen nucleus to the size of a basket ball, the average position of its lone electron would be approximately a two mile walk. Secondly, the orbitals are shown as concentric circles – this isn’t correct in any sense, for two reasons. The first is that electrons don’t orbit the nucleus – orbiting anything requires acceleration, which in turn requires energy. If the electron were to orbit the nucleus they would gradually loose energy and crash into the nucleus meaning life as we know it is destroyed. The second reason, is the approximate shape of an average map of the positions of electrons is only spherical in certain circumstances – that leads us nicely on.
Azimuthal Quantum Number
Isn’t science just the best? Azimuthal. Whilst this number may sound like a number bestowed upon atoms by Zeus. I assure you it is much cooler (sort of). This number is also known as angular quantum number and helps us understand the shapes of each shell. The plural on shape was no error – each shell is broken down into subshells. So for all the electrons in a particular quantum number n, there are a number of different subshells which are helpfully labelled s,p,d,f,g,h,i*… by chemists, or from 1 upwards by normal people. *(This used to stand for sharp, principal, fundamental and diffuse until it was realised this didn’t mean much and it was abandoned)
The natural first curiosity is how do I know how many subshells I will have? For all discussions in this post we assume the atom is in ground state – all of the considerations still exist when the atom is excited, but the electrons will not follow the logical ordering of the lowest possible energy configuration. Let me show you the maximum number of electrons in some subshells and try to spot the pattern
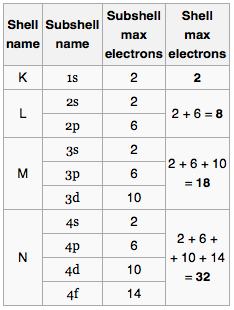
Okay so it isn’t all that simple – but basically for any principal quantum number we can have a maximum of 2n² electrons. Then within a specific subshell;
4ℓ+2
where ℓ represents the azimuthal quantum number – which works providing you label the s subshell as 0, then p as one and so forth.
This may all sound very interesting (or it may not), but why do we care? Well ℓ determines the shape of the orbitals, and when we are trying to visualize an atom it is very hard to do so without knowing the shape. Angular momentum is a function of the inertia a body has along with it’s angular velocity, so it shouldn’t come as too much of a surprise that the angular momentum, or the angular quantum number ℓ dictates what shape the shell should have. If you want to understand why a shell has that shape you are looking at some hardcore mathematics, for which this is not the place. The diagram below shows the various, rather beautiful shapes of orbitals.

Unfortunately the ordering does not obey beautiful logic whereby all of the subshells attached to the principal quantum number 1 are filled first, before the shells attached to n=2 and so on. The s subshell is closer to the nucleus that the p and so on, however there is overlap, which means the outer subshells attached to certain principal quantum numbers are actually further from the nucleus than the inner subshells of higher principal quantum numbers. This means the inner subshells attached to the higher principal quantum number are a lower energy configuration and should therefore be filled first. The following diagram shows you the order in which things should be filled.

Finally let us do our reality check – we are building a clearer picture of what the electron looks like, however the thing which remains potentially at odds with reality is the “shape of subshells”. When we say the shape of a subshell you must not think of this like some kind of plane which the electron is constrained to move in or you will not be looking at reality. Instead think that the electron can go to many different places, however some are more likely that others. Therefore whilst it will go to all places it can go in time, it will visit the most likely ones more often. Now if we could attach some sort of ink to the electron, which left a mark in every place it had been then the final cloud of marker traces would look something like the orbital shapes. That isn’t to say I can tell you where the electron is however – I can tell you where it is likely to be, but due to probability and the uncertainty principle pinpointing that slippery little tyke isn’t possible beyond certain bounds. If you want to get really behind this thing you need to consider the fact that electrons are actually behave like standing waves as they occupy the orbitals – complicated but interesting.
The magnetic quantum number
The final two quantum numbers are shorter to explain so stay with it. The orbital pictures drawn above have three different configurations on the p row, and four on the d row. Whilst it is logical for a human to just have one orientation which is “upright” that is a relative term and nature does not care for human logic. The magnetic quantum number, mℓ, must obey the following inequality where ℓ is the azimuthal number as described above.
−ℓ ≤ mℓ ≤ ℓ
The magnetic quantum number determines which of our orientations the subshells should have – so in the above picture on the p row you can see -1, 0 and +1. Origins are human constructions, but it is interesting and curious that when we do peg down our origin we are constrained for the p subshell to have three orientations, for the d subshell four and so on. It should be no surprise that a spherical orbital therefore only has one possible projection – a feature of the quantum world or human measurement?
The spin quantum number
The spin quantum number is a description of the way in which a particular electron is spinning in an electric field. For an electron ms will be ±½, which you can loosely think of as clockwise and anticlockwise. In physics these two options are called spin up and spin down. Under the Pauli exclusion principle (not for this post) electrons in a particular suborbital cannot have the same quantum numbers – so that means we can only ever have 2 electrons in a suborbital. The spin number is most restrictive! If you are wondering how this works with everything I have just said, look at the p shell in the above diagram. As we discussed you would expect this to hold a maximum of (4 x 1) + 2 = 6 electrons- if you look you will see there are two dumbbell parts to the orbitals plus one you can’t see in the center – it is these which are the suborbitals, so when we say each suborbital can only hold two electrons we mean these – one with spin +1/2 one with spin -1/2. Similarly you can see four suborbitals in the d subshell plus one in the center, which gives the 5 suborbitals for the maximum (4 x 2) + 2 = 10 electrons.
Not that for the magnetic numbers and the spin numbers the values are “random” – there is no set way to know specifically which of these configurations a lone electron is going to choose and there are not universally fixed values – the above are naming conventions which must hold when we perform calculations. I can of course say if I have an electron with spin up, if another joins the party it will have spin down, but as hinted previously these are indeed features of human measurement.

As a mathematician myself, let me try to put it this way. Everything comes under all permutations and combinations with the application of parameters such as physical/chemical properties; energy (electrical, magnetic, etc), mass, gravitational forces, etc. Thank you for making me recall these facts.
LikeLike
No problem at all! I am glad the post got you thinking that way
LikeLiked by 1 person
Thanks for the information. Maybe I can somehow use this information to stop my brain popping in and out of existence like the virtual particle it has become.
LikeLike
No problem at all! Let’s hope so!
LikeLike
Reblogged this on Lost Dudeist Astrology.
LikeLike
Reblogged this on Still Another Photoblog.
LikeLike
Pingback: Quantum Numbers through Universe Exploration >>>>>>>>>>>>>>>>>>> – divyanshspacetech·
When we heat up a bar of iron we are told the atoms begin to vibrate faster does this include the electrons ? When the bar cools heat waves are radiated , in fact if the bar goes red it emits light , is this the electrons moving down into lower orbits?
Why is it that lead a large atom melts at a low temperature whereas iron a much smaller one melts at a much higher temperature?
Do the outer electrons in the larger lead atoms make them repel each other so the bonding is loose?
Is it true that at absolute zero all motion ceases, even atomic motion?
LikeLike
Hi Kertsen,
So yes when we heat up a bar the individual atoms do indeed move faster; this does include the electrons but note that what the electrons will do is move to higher orbitals. This is what I was referring to when I said that all of the discussion were in regard to an atom in ground state. When an electron returns to a lower orbital, this orbital has a lower energy and the difference is given out in radiation – the greater the frequency the more violet the color assuming this is radiation we can see. It is the interesting part of quantum mechanics that only discrete amounts of energy can be absorbed and emitted – not any amount.
With regard to lead melting at lower temperatures than iron, generally speaking yes this can be seen as a phenomena of size but there are a few more details. Firstly you must ask if your bonding is ionic or covalant in nature. Ionic is stronger as it involves the attraction of ions with opposite charge. In covalent the strength is generally determined by the size of the atom, as the larger the atom the stronger the transient dipoles that can be created. With metals the main source of metalic bonding is attraction between the free valence electrons and the then positive metalic ions – so the more free electrons you have the tighter the boding. This can typically get the atoms to line up in sheets which gives metal many of its properties. So if you think of electron as glue – the more glue you have the stronger the hold.
In terms of absoloute zero there is still a base level of jiggling – quantum mechanics makes it so (uncertainty principle)
LikeLike
Great Information
LikeLiked by 1 person
Why users still use to read news papers when in this technological world all is
existing on web?
LikeLike
Reblogged this on EDC Writing and commented:
As a scientist and a physical chemist at that, I couldn’t resist posting as a reblog this … reminds me of what I’m supposed to know in such a well written and elegant way …enjoy!
LikeLike
Quantum number is a bit redundant though, isnt it? (Im being REALLY pedantic now lol). Integers (which for most mean the entirety of numbers, or worse just Naturals) are quantum by definition!
As an aside, the orbitals always seemed strange to me, but now they are quite simple in why electrons choose that shape of their probably location.
LikeLike
The shape of the orbitals totally mystified me for a while too! It was only when I really started getting behind angular momentum and such things that I began to appreciate, although I cannot fully derive the shape of the orbitals myself yet the mathematics is far too grizzly!
LikeLiked by 1 person
I looked over the formulas (in a physical chemistry grad text book) and its insane. It’s actually very simple with the research I’m doing, but I have to re do it! lol. I was focused on prime numbers (not just the twin prime conjecture, but in fact a formula that includes all primes) so I lost the scribbling with the orbital general formula , that covers all of them.
Just to make this note, I didnt set out for the primes (its a fools errand in itself) but I came across some very interesting things by chance (long story, it invovles a surgery, and a morphine IV lol)
LikeLike
Reblogged this on MARSHALL W THOMPSON, SR and commented:
Brain Food! Peace, Marshall
LikeLiked by 1 person
Pingback: The rise of the neutrino | Rationalising The Universe·
Pingback: The rise of the neutrino·