Today I want to talk about electron configuration because I think it is one of the biggest failings of the education system. It is widely accepted in schools that we cannot be taught the full truth about things from day one – and I accept this in full. However what I do not accept is when in order to teach something a misleading version is taught first because the issue with this is if that student does not go on to further studies then they spend the rest of their life in the dark. This in my view is what happens with electron configurations, something I will outline in this post.
You are first taught that electrons orbit in a planetary model in a configuration that goes 2 in the first shell, then 8 in the shell after that and so on and so forth. What I am going to explain now is a simplified version of reality – the version of reality I just explained is beyond even considering how simplified it is. I will however explain towards the end why despite being terribly oversimplified it does work in terms of allowing you to do some Chemistry – this is why it had you so fooled all this time.
The single most important notion when you try to comprehend an electron configuration is the quantum number – there are two which we are going to concern ourselves with in order to understand the configuration and these two are used by both Physicists and Chemists widely. The last two we will state but not explain.
The principle quantum number is the number which is most familiar two you – it is the shell (or orbital) numberings of the electrons orbiting the nucleus which can range from 1 until theoretically infinity – but clearly there are natural limits in terms of the orbitals of the heaviest known elements. So the shell numbering in itself is quite easy – it goes 1 for hydrogen and helium, two for lithium, beryllium, boron carbon… 4 for potassium… 5 for tin… notice anything? Take a look at the periodic table.

What you should see is that the principle quantum number corresponds to the period number. So if you have a periodic table you can very easily tell me the principle quantum number for any element – very good so far. Now when you have you principle quantum number that also allows you do construct quite easily the number of electrons that can be found in that level. The relationship is easy – if we denote the principle quantum number n then the number of electrons that can be found at that particular given level is 2n². So for level 1 we have 2 – which is fine we had that before. And then for level 2 we have 2 x 4 so great the same as before again we have 8. But what about level 3? We have 18. That isn’t 2 and it isn’t 8… it is 18. As we start to go up and up the number starts to get bigger and bigger. Much bigger. So what is going on?
This is where we need our second quantum number. If you only looked at the principle quantum number then trust me the world would not make very much sense because electrons do not occupy shells in the order of 1 then 2 then three. It turns out, within each of these shells are subshells. In fact the number of subshells at each level is very easy to work out too – the number of subshells is equal to the principal quantum number. So shell 1 has no subshells (1 subshell is just the shell itself). Number two has two subshells and so on and so forth. Before we delve any further it is worth quickly stopping and saying what we really mean when we say “shells”.
You may well be familiar with the uncertainty principle? Well if you are not all it basically says is you cannot determine an electrons exact location and velocity at the same time. The basic issue that falls out of this is I cannot tell you exactly where my electrons are. What I can tell you is where they might be. Actually I can do better than this, I can tell you where they will probably be. So what I can show you is a probability distribution of where my electron is most likely to be and it looks a little bit like this.

The above graph is for one given subshell – so what we find is that the probability of finding the electron is greatest at a certain distance from the nucleus. Then we define the space that this subshell occupies as the region where there is a 95% chance of finding my electron – i.e. the region where it probably is. So when people say that electron shells are more like fuzzy clouds this is why – the electrons could in theory be anywhere – the graph is a asymptotic to the x axis, it is just they tend to move within the confines of a certain area of space.
Hopefully none of this is too taxing so far – personally I think it isn’t too difficult to visualise. What we need to consider next is the single most important part. What makes an electron a member of one shell, and not a different shell? Any two electrons have the same negative charge and the same mass and within a given atom are influenced by the same nucleus. So what should make an electron favour certain locations in one subshell but different locations in another? The answer to all of this is energy levels. Each subsell represents a different energy level. Remember that electrons are attracted to the nucleus – they favour positions which are closer to the nucleus with lower energy levels. The only reason that they do not all go into the first subshell is that they cannot fit (this makes sense if you think about it – the subshell occupies a certain amount of space, and each electron is negatively charged so you can only fit a certain number of particles that repel each other into a given region of space.
Okay, I think we are ready to tie this all together. We have the principle quantum number. This is the shell number. For any given shell n, there are n subshells all representing orbitals of different energy levels. Now we give these subshells a quantum number – the azimuthal quantum number. If you are a chemist you will know these as s, p, d, f, g, h etc in that order – and these we will stick with for now. Often in Physics we use different notation but it is actually harder to type on a keyboard. Now if this azimuthal quantum number is ℓ, then the relationship of how many electrons can be in each subshell is;
2(2ℓ + 1)
So it is easy – s can have 2, p can have 6, d can have 14 etc. So you might think the logical order is 1s,2s,2p,3s,3p,3d etc but this is wrong and the reason for that is that electrons prefer naturally the lowest energy levels – and actually 3d is a higher energy level that that which is found with 4s. So the result is that the 4s subshell is formed first. There is a way for simple subshell configurations you can work out the final shell from the periodic table and work backwards – but for now just take this as fact:
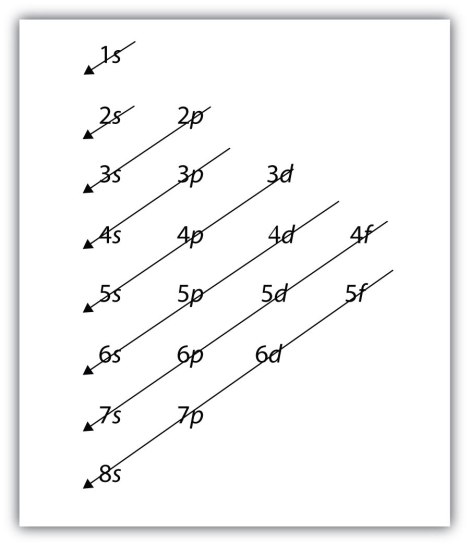
So we have the 4s filled here before the 3d and so on and so forth. Are you starting to understand a little bit now why the electrons are viewed more as fuzzy clouds that as strict orbital rings? It really does make much more sense. When you know the number of electrons in an atom (fairly easy to work out) you can write out the electron configuration which looks like this, for the element oxygen:
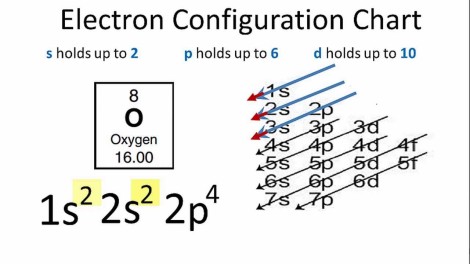
Now if you remember anything from Chemistry you will remember that this configuration isn’t fully “stable”… what we really mean by this is it isn’t the desired state for oxygen is to complete its outer shell – the p shell which holds up to 6. Now remember I said I would explain why the old 2,8,8 model often works? It is because for the majority of elements the Chemistry is dominated by the outer shells – the p and s shells which together have 8 electrons as a maximum. So when you were saying you needed to complete the 8 electrons, you were in a way flirting with the truth. But because the understanding is too simplistic you cannot fully appreciate what happens with more complex electrons.
Understanding the electron configuration is really everything – there is nothing much more to Chemistry. Why is Oxygen found as O2? Well because it needs two electrons to complete its outermost p-shell and in order to do this it can “share” two electrons (i.e. they can occupy a region of common space) and complete the shells. This is a pure covalent bond with no polar element.
Couple of closing remarks:
- The spin forms another quantum number and is very important in physics. The first subshell of 2 must have electrons of opposite spin.
- There is a further quantum number that determines the magnetism which along with the azimuthal quantum number determines the shape of the orbital.
The shape of the orbital I have displayed below for interest only. How do we know this? VERY HARD vector mathematics which I do not profess to understand. Maybe I will update you with more information when I have taken my final year quantum mechanics module. None the less the shapes are quite beautiful functions in three dimensional space. This is for 1s, 2p, 3d, 4f.

Thanks for reading and being patient with me this month – let’s just say it has been a busy one!

Reblogged
Thank you for your support and leadership
LikeLiked by 2 people
Thank you very much Sir
LikeLiked by 1 person
Pingback: #Electron #configuration #demystification @barkinet #fb – Engineer Marine Skipper·
Reblogged this on things I've read or intend to.
LikeLiked by 1 person
Well written! Explained in a Crystal clear manner! Took me back to the times when I had to consult JD Lee just to get a hang of what this all was about 😊
LikeLike
Thank you very much for your support 🙂 I appreciate it
LikeLike
Very well written post, I have been planning to write something about orbitals too, this made me more motivated!
LikeLiked by 1 person
Thank you very much! I look forward to your post… To be honest I have only just started to fill in some of the gaps in my knowledge of the atom but it is wonderful. Things like covalent bonding just made no sense until I started to view it this way
LikeLiked by 1 person
You are welcome!
LikeLiked by 1 person
This is kind of explained in Alevel Chemistry! Crazy to think this is still a simplified version… Then you start to get Schrodingers equation and things get a bit crazy haha…
LikeLike
Yes my Chemistry stopped at GCSE until now! Really enjoying catching up… I had picked up on some things through Physics but it completes the picture. Personally I think it should be taught this way – always. I think it is patronizing to think kids who can cope with the idea of an electron cannot cope with the idea of filling subshells in a certain order… perhaps leave out Schrodinger though.. gives me a headache thinking about it
LikeLiked by 1 person
This is a very clear explanation, thank you. Fortunately I had an excellent A-level chemistry teacher who didn’t over-simplify it for us: I do think it actually makes it clearer this way.
LikeLiked by 1 person
I could not agree more, it really is a beautiful area of science. Thank you for your comment
LikeLike
Pingback: A Spectacular Show | Rationalising The Universe·
Pingback: A Spectacular Show·